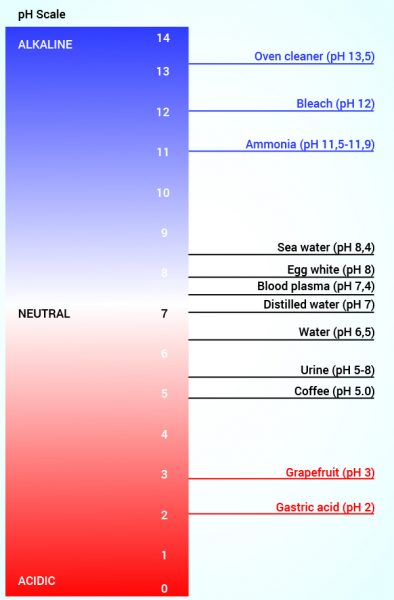
When we talk about acids and bases we generally use the pH scale. This is a scale that indicates how acidic or alkaline something is. The pH scale is a logarithmic scale and ranges from 0-14, with zero being the most acidic and 14 the most alkaline. When the pH is 7, we say that the solution is neutral. Liquids with a pH between 0 and 7 are thus called acidic solutions (excess of H+), and liquids with a pH above 7 is called an alkaline solutions (an excess of OH–).
A mixture of an acid and a base will neutralize each other, but the pH does not necessarily become neutral, because it depends on the amount of acid and/or base that finally determines the pH value. If the amount of H+ and OH– is equal, the solution becomes a neutral because these ions bind with each other to form water (H+ + OH– → H2O).
In fact water itself is very slightly acidic but we wont go into the details of this here.
The pH scale is logarithmic – this means that between each unit of one on the scale, there is a factor of 10 in the difference in the concentration of hydrogen ions. That is, pH 5 is 10 times more acidic than pH 6 and pH 4 is 100 times more acidic pH 6.
Regulation of acid-base balance, i.e. the pH, is vital for the organism. Enzymes and biochemical processes works optimally at certain pH values, and at worst can be destroyed by non-physiological acid-base level in the body fluids. The pH varies slightly between different fluid compartments of the body. In arterial blood, the pH is 7.4, in venous blood and in the interstitial fluid, the pH is 7.35, while the average intracellular pH is 7.0. In the respiration physiology unit , you learned that venous blood contains more CO2 than arterial blood, and that there is a direct correlation between CO2 and pH. The more CO2, the lower the pH. This explains the difference of pH in the arterial and venous blood.
In the stomach, the pH is between 1.5 and 3. This is up to 100,000 times more acidic than blood.