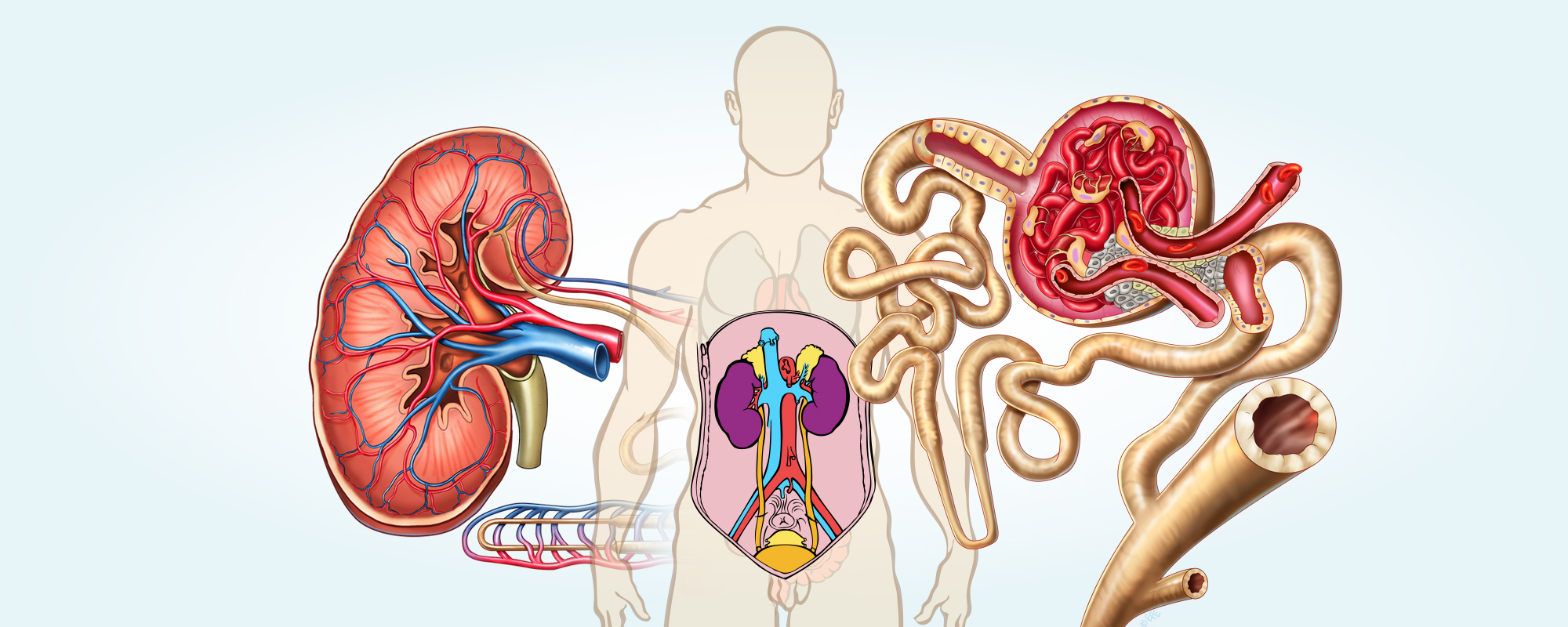
The body consists of about 60% water. Body fluid is distributed in three different fluid compartments: plasma, the extracellular space and the intracellular space. In these fluid compartments there are different substances: salts, sugars, amino acids and various other acids, and various proteins. But the substances are unequally distributed in the various compartments. Since the body is constantly changing its fluid and salt levels through sweat, faeces and urine and through ingestion of water and food, the body content of water and salts are carefully regulated and controlled.
The definition of an acid is a substance that can release hydrogen ions (H+). It is free hydrogen ions not bound hydrogen ions that determine the acid levels in the blood so the more free hydrogen ions the lower the pH of the blood. One of the body’s most important molecules that bind with hydrogen ions are bicarbonate (HCO3–) ions. Acids builds up in the body as a result of metabolism and nutrient absorption through the intestine. It is important to keep the pH of the blood and body tissues within certain limits and of the availability of bicarbonate is thus important in the regulation of the body’s acid level.
The kidneys play an active and important role in both the water, salt and acid – base regulation in both the blood and body tissues.



